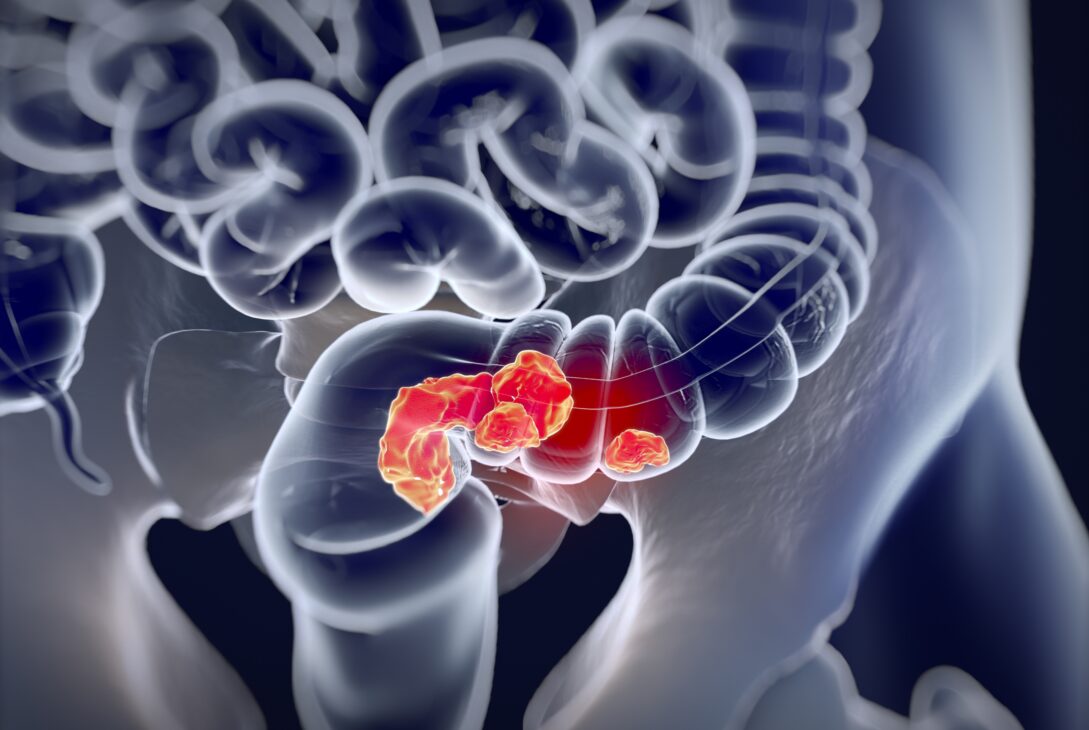
Traditionally, colorectal cancer screening has been both time-consuming and invasive. Individuals must first prepare using a bowel-cleansing solution before reporting to your physician for a colonoscopy, an invasive procedure that uses a long, flexible tube inserted through the rectum to check the colon. However, this screening is necessary and important. During screening, doctors can remove any precancerous polyps before they turn into cancer. If the cancer has already formed, early detection plays a huge role in outcomes.
Yet many people find themselves eschewing colorectal cancer screenings, even though younger people are getting colorectal cancer at rates higher than the historical average. Why is this? Some people feel there is a stigma to getting a colorectal cancer screening. Others might feel embarrassed, afraid, or uncomfortable with the procedure’s invasiveness at the hands of a physician. In fact, up to 44 million American adults remain unscreened each year.
This is where ColoSense comes in. Katie Adams reports on the first noninvasive colorectal cancer screening test in MedCityNews, where she shares that the FDA has approved ColoSense as a breakthrough device. Commercialization should happen by the end of 2024 or in early 2025. ColoSense, developed by Geneoscopy, is the first RNA-based molecular screening test to qualitatively detect colorectal cancer and advanced adenomas in average-risk individuals (those ages 45+ with an average risk of developing this form of cancer). As the ColoSense website explains:
Colosense is a multi-target stool RNA (mt-sRNA) test engineered for the qualitative detection and stabilization of RNA signals. [It uses RNA biomarkers that] provide a dynamic view of disease activity, not subject to age-related methylation patterns that can lead to variability in test performance across different age groups.
The test generates a score using a stool sample that suggests whether someone could possibly have precancerous polyps or cancer; if the test suggests this is likely, it also encourages the person to set up an appointment with their physician for more invasive testing. Alternately, if the test shows that they do not have polyps or cancer, that person can follow the recommended screening guidelines.
Editor’s Note: Get Involved
Cancer doesn’t discriminate. WHATNEXT and its partners are interested in amplifying the voices of those from all identities and backgrounds. If you have a cancer journey to share, reach out here to learn more about how your voice can help spread awareness and inspire individuals from all walks of life.
The Facts on Colorectal Cancer
Colorectal cancer often begins as benign polyps outside of the colon which turn cancerous over time. It is one of the leading causes of cancer-related death across the globe, contributing to hundreds of thousands of deaths each year. If you are Black, overweight, over 50 years old, have a family history of colorectal cancer, smoke or drink alcohol heavily, have diabetes, or eat a low-fiber and high-fat diet, your risk of this cancer is higher.
Many people are asymptomatic (do not have symptoms) in early stages of the disease. When symptoms do appear, they can include losing weight without trying, a persistent change in bowel habits such as chronic constipation or diarrhea, feeling like your bowels haven’t emptied completely, rectal bleeding, bloody stool, fatigue and general malaise, abdominal pain or discomfort, narrow stools, and anemia (having low red blood cells). Colorectal cancer screenings can identify cancer before it progresses, giving you the chance for early diagnosis and treatment, which correlates with better outcomes.
cancer screening colon cancer colorectal cancer ColoSense diagnostics FDA approval oncology rare cancer
Last modified: May 31, 2024