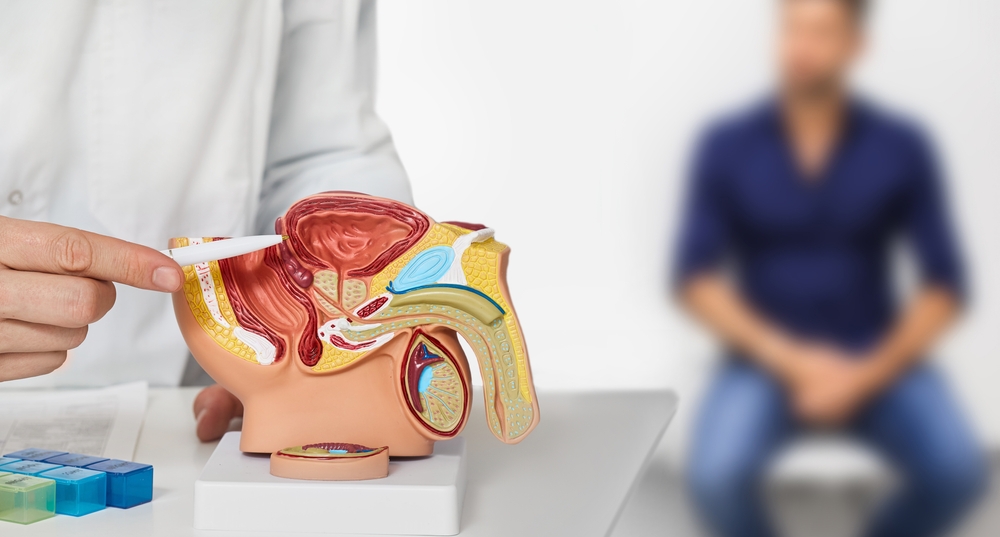
Many early-stage prostate cancers need testosterone to grow and spread. Castration-resistant prostate cancer refers to prostate cancer that continues to grow even when testosterone levels are at or below castrate level (AKA – your testosterone levels are very low). Approximately 20% of people with prostate cancer have castration-resistant prostate cancer.
People with this cancer may experience symptoms such as extreme fatigue, unintentional weight loss, bone pain, shortness of breath, difficulty urinating and/or painful urination, or hematuria (blood in the urine). Symptoms may depend on how large the initial tumor is, as well as where the cancer has spread to.
According to Jen Broghan in PharmaTimes, men in Scotland with metastatic castration-resistant prostate cancer (metastatic meaning it has spread) now have a new treatment option: Lynparza (olaparib). The Scottish Medicines Consortium (SMC) approved Lynparza in March 2024. The treatment is approved for use in conjunction with either prednisolone or abiraterone and prednisone.
This combination treatment offers a benefit to patients. Many therapies for prostate cancer, including the current standards-of-care, focus on androgen deprivation therapy. However, given the physiological and biological nature of castration-resistant prostate cancer, the standards-of-care do not typically work for this specific patient population.
What is Lynparza?
Lynparza is a PARP inhibitor. It inhibits PARP proteins to stop cells and tumors with mutations and issues from repairing themselves, essentially stopping the cancer from spreading. Outside of the recent approval in prostate cancer, Lynparza is approved to treat fallopian tube cancer, advanced ovarian cancer, and primary peritoneal cancer with germline or somatic abnormal BRCA genes. It also has some use in treating breast cancer and pancreatic cancer.
Lynparza’s recent approval in metastatic castration-resistant prostate cancer follows data from the Phase 3 PROpel clinical study. During the study, researchers examined how safe, effective, and well-tolerated this treatment was when used alongside abiraterone. The research team found that this combination treatment halted disease progression and lowered mortality risk by around 34%.
castration-resistant prostate cancer drug approval metastatic prostate cancer oncology prostate cancer
Last modified: April 22, 2024